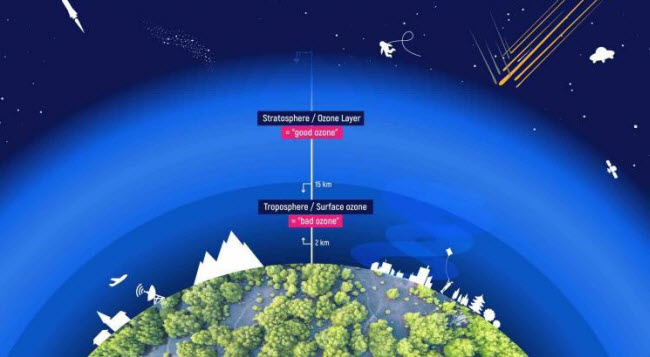
Ozone is a blue gas with a distinctive, chlorine-like odor, composed of three oxygen atoms. It is highly reactive with other compounds and forms in two primary ways. Naturally, it results from various chemical reactions occurring high in the atmosphere, creating a thin layer known as the ozone layer in the stratosphere. This layer acts as a protective shield for our planet by absorbing harmful ultraviolet (UV) rays from the sun and preventing them from reaching Earth. The second method involves industrial production for various commercial purposes, though it is used in lower concentrations due to its classification as a pollutant that can harm humans, animals, and plants.
The discovery of ozone dates back to the 18th century. In 1785, Dutch chemist Martinus van Marum conducted experiments involving electrical sparks over water, noticing an unusual odor he attributed to electrical interactions. He failed to recognize that he had produced ozone. It wasn’t until half a century later that German chemist Christian Friedrich Schönbein identified the same pungent odor as the scent often associated with lightning. In 1839, Schönbein successfully isolated this gaseous substance and named it “ozone,” derived from the Greek word “ozein,” meaning “to smell,” due to its distinctive odor often detectable after thunderstorms. However, the chemical formula (O3) was not determined until 1865 by Swiss scientist Jacques-Louis Soret.

In the latter half of the 19th century and into the 20th century, this gas was considered a beneficial environmental component by naturalists and health researchers, who often regarded high altitudes as advantageous due to their ozone content. However, by 1873, scientists James Dewar and John Gray McKendrick began observing its harmful effects. They documented that frogs grew slowly in its presence, birds struggled to breathe, and rabbit blood showed low oxygen levels after exposure to ozone-treated air. Schönbein himself reported health issues from inhaling ozone. During World War I, it was tested in London as a potential wound disinfectant, where it was applied directly to wounds for 15 minutes, resulting in damage to both bacterial cells and human tissues. This led to its dismissal and the search for other sterilization techniques due to its harmful effects and potential lethality at high concentrations.
Physical and Chemical Properties
Ozone is a colorless or pale blue gas, slightly soluble in water and more soluble in inert non-polar solvents like carbon tetrachloride or chlorofluorocarbons. It turns blue at −112°C, and its liquid form is hazardous as both concentrated gaseous and liquid ozone can explode at temperatures above −193.2°C.
Ozone is formed from the combination of molecular oxygen (O2), which is the natural oxygen we breathe, with another oxygen molecule to produce O3. It naturally occurs in the stratosphere, a layer of the atmosphere ranging from 10 to 50 kilometers above Earth’s surface, with its peak concentration at 32 kilometers in a region known as the ozone layer. This layer protects Earth by absorbing harmful UV rays from the sun, making life on Earth possible.

Ozone Depletion
Ozone is relatively unstable and can be destroyed by molecules containing nitrogen, hydrogen, chlorine, or bromine. These molecules work to separate the third oxygen atom from the ozone molecule. Since the 1950s, scientists have measured ozone concentrations over Antarctica, which first hinted at ozone layer problems. By the 1980s, researchers mapped a hole in this layer, later confirming that human-made pollutants called chlorofluorocarbons (CFCs), used in industrial processes like refrigeration and fire suppression, were the cause. Chlorine and bromine atoms in CFCs are ozone-depleting, with one chlorine atom capable of destroying over 100,000 ozone molecules in the stratosphere, according to the U.S. Environmental Protection Agency (EPA).
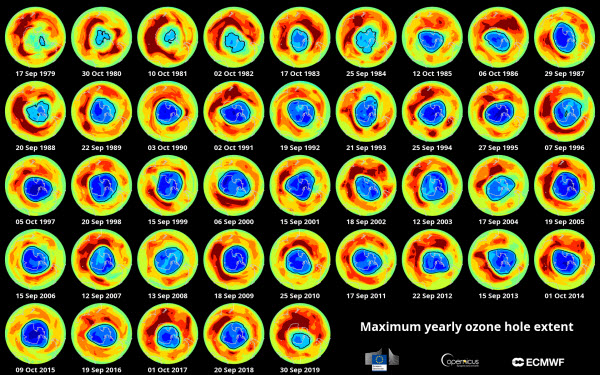
Further research revealed that ozone depletion was not limited to Antarctica but also occurred in various global regions. This led to the 1987 signing of the Montreal Protocol on Substances that Deplete the Ozone Layer, an international agreement committing signatories to phase out CFCs and other harmful pollutants. These efforts have borne fruit, as a recent study showed a 20% reduction in ozone depletion from chlorine between 2005 and 2016. By 2019, the Antarctic ozone hole had shrunk to its smallest recorded size since its discovery. However, in 2020, scientists observed that the Arctic ozone hole, though rarely opening, was larger than the Antarctic one, raising concerns. This unprecedented event ended after two weeks, and researchers are still uncertain if it indicates a new trend.
Ozone Pollution at Earth’s Surface
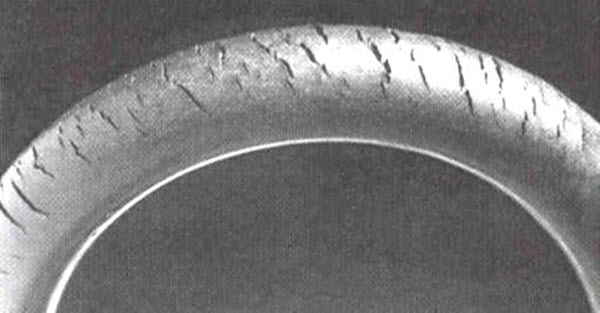
Near the Earth’s surface, ozone can be harmful and is known as smog. It forms from nitrogen oxides emitted by vehicles, power plants, industrial boilers, refineries, and chemical factories, reacting with other organic molecules in the atmosphere. Inhalation of ozone can cause chest pain, throat irritation, coughing, and damage to lung tissues. This is particularly dangerous for children, the elderly, and those with respiratory conditions such as asthma, emphysema, and bronchitis. It also affects plants, impacting forests, parks, and wilderness areas, and can degrade rubber.
Due to ozone’s risks, it is listed as a common pollutant under the U.S. Clean Air Act. The EPA provides an online air quality tool to alert people when pollution levels, including ozone, are high in their area. The Centers for Disease Control and Prevention (CDC) recommends staying indoors when ozone levels are high, reducing strenuous outdoor activities, and planning outdoor activities in the morning or evening when ozone concentrations are lower. The EPA has established national air quality standards aimed at reducing surface ozone levels by limiting pollutants from vehicles and factories, promoting walking, biking, and public transport, and reducing air conditioner use when ozone levels are high. They also encourage finding ways to make homes more environmentally friendly.
Industrial Applications of Ozone
Ozone is produced industrially using devices called ozone generators. It is used in various industries, including pharmaceuticals, synthetic lubricants, and bleaching agents, and to kill microorganisms in air and water sources. Some municipal drinking water systems use ozone to kill bacteria instead of the more common chlorine. Lower levels of ozone are used as a disinfectant in residential homes, hospitals, food processing plants, and swimming pool sanitation. Water-intensive industries, such as breweries and dairy farms, use dissolved ozone effectively as an alternative to chemical disinfectants like peracetic acid or heat. Ozone is also employed to kill insects in stored grains and for other applications.